Satbility Guide For Regulatory Affairs
💠Stability study ICH guidelines
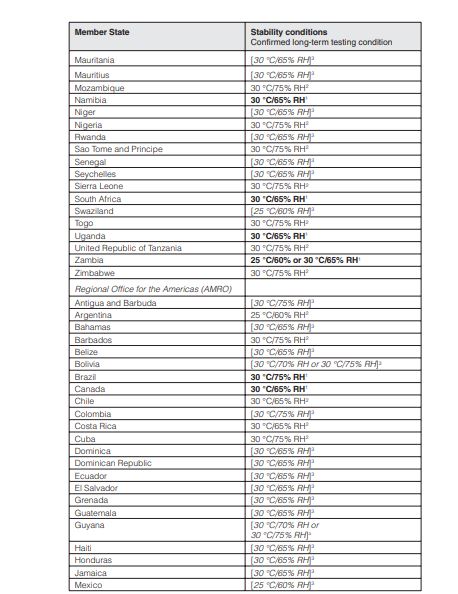
💢 stability zones conditions in details for long term, intermediate & accelerated stability testing
💨3.2.P.8.2 Post-approval Stability Protocol & Stability Commitment
⚡ A written commitment (signed & dated) is submitted in the following cases:
Results of stability studies on three production batches are not completed for all the intervals till the proposed shelf-life period.
⚡ Data from stability studies on less than three production batches.
⚡ Stability data are not on production batches.
💨3.2.P.8.3 Stability Data
⚡Results of the stability studies should be presented in a tabular format. The results of all testing parameters related to each batch for the entire testing period should be presented in one table (i.e. presenting the results of one parameter of all batches in one table is not acceptable).


storage services supporting your drug product development, commercial stability
studies, batch release and quality control testing
studies, batch release and quality control testing
ICH pharmaceutical stability studies are an essential component of the development and lifecycle of pharmaceutical products, in particular, supporting the development process and IND / NDA
submission activities. They allow evaluation of active pharmaceutical ingredient (API) stability or drug product stability under the influence of a variety of environmental factors such as temperature, humidity and light. Data from these studies enable recommended storage conditions, retest intervals and shelf lives to be established.
submission activities. They allow evaluation of active pharmaceutical ingredient (API) stability or drug product stability under the influence of a variety of environmental factors such as temperature, humidity and light. Data from these studies enable recommended storage conditions, retest intervals and shelf lives to be established.
Selecting an experienced
stability study service partner who offers efficient study management, flexible storage conditions and testing capabilities which satisfy all regulatory criteria for your real-time, accelerated or forced-degradation study requirements is key to success. Stability testing can present significant analytical hurdles and specialised knowledge is required to develop and validate stability indicating methods and perform analysis of leachable substances which migrate from pharmaceutical packaging into the product.
stability study service partner who offers efficient study management, flexible storage conditions and testing capabilities which satisfy all regulatory criteria for your real-time, accelerated or forced-degradation study requirements is key to success. Stability testing can present significant analytical hurdles and specialised knowledge is required to develop and validate stability indicating methods and perform analysis of leachable substances which migrate from pharmaceutical packaging into the product.
With a network of ICH stability storage facilities in the UK, US
and Australia, we offer an extensive capacity and a range of conditions including climatic walk-in chambers, cabinets and refrigerated as well as freezer storage which are fully controlled and monitored with back up chambers at each site. All sites have 24-hour alarm systems with dedicated on call teams to react to the excursions from storage conditions. Our stability teams provide professionally managed Good Manufacturing Practice (cGMP) stability programs for even the most complex of dosage forms, APIs or product types including orally inhaled and nasal drug products (OINDP), biopharmaceuticals, consumer healthcare, medical devices or vaccines. We also offer a responsive and bespoke stability contingency and disaster recovery storage service to help you mitigate the risks associated with costly stability trials.
and Australia, we offer an extensive capacity and a range of conditions including climatic walk-in chambers, cabinets and refrigerated as well as freezer storage which are fully controlled and monitored with back up chambers at each site. All sites have 24-hour alarm systems with dedicated on call teams to react to the excursions from storage conditions. Our stability teams provide professionally managed Good Manufacturing Practice (cGMP) stability programs for even the most complex of dosage forms, APIs or product types including orally inhaled and nasal drug products (OINDP), biopharmaceuticals, consumer healthcare, medical devices or vaccines. We also offer a responsive and bespoke stability contingency and disaster recovery storage service to help you mitigate the risks associated with costly stability trials.
GMP Stability Services:
cGMP registration stability programs
- Protocol design and program management
- Development and validation of stability indicating methods
- Stability testing for APIs, clinical trial materials, formulated products
- Tailored reporting (timepoint and final reports)
- Temperature cycling, freeze-thaw and shipping studies
- Stability contingency and disaster recovery storage
- Forced degradation testing
- Real-Time stability testing
- Accelerated stability testing
- Formulation stability testing
- Biologics stability studies
- Specialist expertise for OINDP stability programs
- Extractables / leachables
ICH Stability Conditions Available
We have an extensive range of ICH stability conditions available to our customers across the
We have an extensive range of ICH stability conditions available to our customers across the
- 25ºC / 40% RH
- 25ºC / 60% RH
- 30ºC / 25% RH
- 30ºC / 35% RH
- 30ºC / 65% RH
- 30ºC / 75% RH
- 40ºC / 75% RH
- Cabinets 50 ºC, 57 ºC, 60 ºC
- Storage at 2-8ºC, -20ºC, -40ºC, -80ºC
- Photostability (ICH Options 1 & 2)
- Specialised conditions
- Freeze/thaw cycle tests
Stability Testing and Analysis
Our analytical laboratory network provides development and validation of stability-indicating methods utilizing a range of technologies to identify and quantify degradation products. Routine time point testing includes the usual tests such as assay and impurity analysis, dissolution, moisture, hardness, friability and disintegration scientists have specialist expertise in OINDP testing for stability including particle or droplet size to provide data to drive understanding of the delivered formulation and delivery of the drug from the inhalation device. Our know-how
in extractables and leachables studies means that we can ensure that the product container/packaging system demonstrates sufficient stability over the relevant lifecycle of your product.
Our analytical laboratory network provides development and validation of stability-indicating methods utilizing a range of technologies to identify and quantify degradation products. Routine time point testing includes the usual tests such as assay and impurity analysis, dissolution, moisture, hardness, friability and disintegration scientists have specialist expertise in OINDP testing for stability including particle or droplet size to provide data to drive understanding of the delivered formulation and delivery of the drug from the inhalation device. Our know-how
in extractables and leachables studies means that we can ensure that the product container/packaging system demonstrates sufficient stability over the relevant lifecycle of your product.








Awesome Blog, good information shared. cover all the the things in short heads off dude keep doing
ReplyDeleteThanx for appreciation
DeleteNice information shared. keep doing
ReplyDeleteGood knowledge sharing
ReplyDeleteThanks for sharing my slides, wish it will support you on reaching the important points related to stability study
ReplyDelete